BCC
2.8300


Olivier Goy is running out of time.
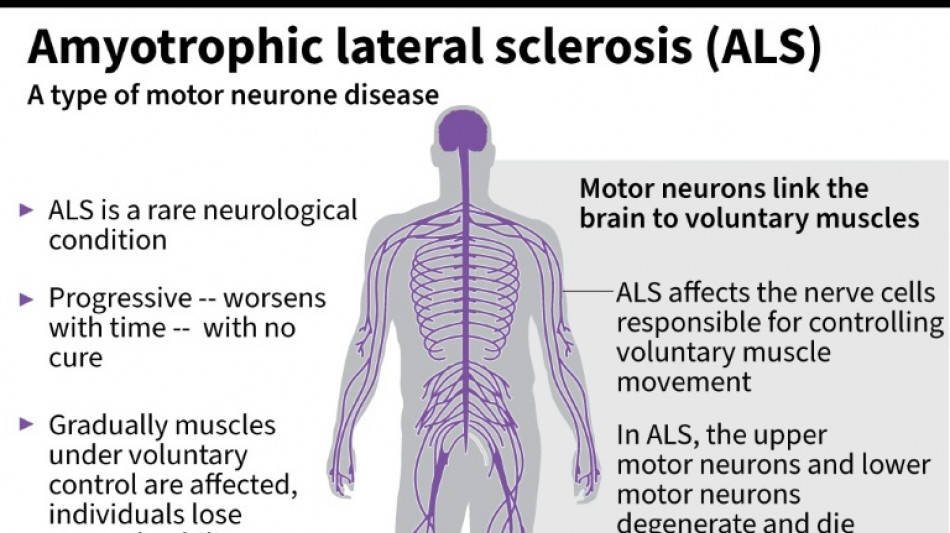
The French entrepreneur was diagnosed in 2020 with amyotrophic lateral sclerosis (ALS) -- the incurable neurodegenerative disease that normally claims the lives of patients within three to five years.
There are new treatments that have given patients hope of being able to extend their lives by an invaluable few months, but the approval process in Europe is taking time, infuriating desperate patients.
"When you are certain to die soon, patients and some doctors are ready to take some risks," Goy told AFP.
In response to the lack of new treatments in his native France, the founder of the fintech start-up October spends 3,000 euros ($3,180) every month to buy the ingredients to make his own drugs.
ALS, also known as Lou Gehrig's disease, attacks the motor nerve cells in the brain and spinal cord, progressively paralysing muscles until patients cannot walk, eat, speak or breathe.
Around one in 10,000 people have the disease in the EU, according to the European Medicines Agency.
The drug Riluzole, which has been available in Europe and the UK since the 1990s, is capable of prolonging the lives of patients by around three months.
But otherwise, no new treatment has been approved in Europe for more than two decades.
- 'First hope in 20 years' -
A new treatment called AMX0035 was given the green light in the United States and Canada last year.
"It is the first hope we have had in 20 years: the first drug which is aimed at everyone and which had results" suggesting up to six months in added life expectancy, said Sabine Turgeman, head of the French Association for Research into ALS.
But the extent of the benefits of AMX0035 remains unclear. The US Food and Drug Administration approved the drug, sold under the name Relyvrio, based on the results of a single Phase 2 trial that involved just 137 participants.
The drug's developer, Amylyx Pharmaceuticals, is conducting larger, more comprehensive trials, with results expected in 2024.
Amylyx said earlier this month that the European Union's drug watchdog EMA is reviewing its submission for approval and it expects a decision in the first half of this year.
But for those with the disease, every delay represents a significant amount of the time they have left.
"It's not going fast enough," Turgeman said. "This disease is not on bureaucratic time".
For European patients who cannot afford to import their own ingredients like Goy, the only way to get access to new treatments is to join a clinical trial.
But such trials have very specific criteria for selection -- and even if a patient gets in, there is a chance they will be in the group given a placebo.
- 'Totally abandoned' -
Given how swiftly the disease progresses, patients and families are pressing for more options.
"We feel totally abandoned," said Sophie Garofalo, whose brother was diagnosed with ALS five years ago.
His family tried to enter him into clinical trials, "but either he does not meet the criteria, or the trials have already started," she said.
"He is ready to take anything, try everything".
French pharmaceutical company AB Science is developing another potential treatment using the drug masitinib, which initial results suggest could add months to the lives of patients.
The firm's CEO Alain Moussy said that because "time is very limited" for ALS patients, there should be more flexibility in the approval system.
"What degree of risk should be taken? That's for the health agencies to answer -- but they can guided by policymakers and patients," he said.
S.Ogawa--JT